Pergunta
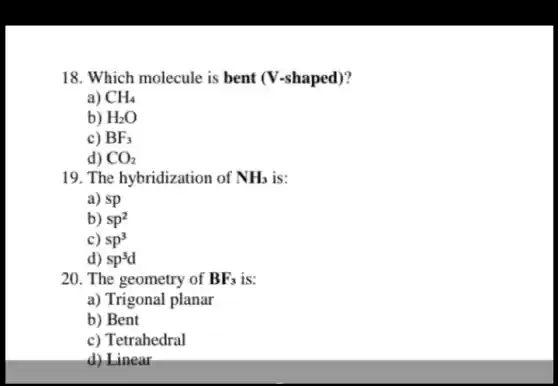
18. Which molecule is bent (V-shaped)? a) CH_(4) b) H_(2)O c) BF_(3) d) CO_(2) 19. The hybridization of NH_(3) is: a) sp b) sp^2 c) sp^3 d) sp^3d 20. The geometry of BF_(3) is: a) Trigonal planar b) Bent c) Tetrahedral d) Linear
Solução
 Verification of experts
Verification of experts4.5316 Voting
Renata MariaProfissional · Tutor por 6 anos
Responder
18. The correct answer is b)
19. The correct answer is c)
20. The correct answer is a) Trigonal planar. The geometry of
. The molecule
has a bent or V-shaped geometry due to the presence of two lone pairs of electrons on the oxygen atom. The lone pairs repel the bonded hydrogen atoms, resulting in a bent shape.
19. The correct answer is c)
. The hybridization of
is
. In the
molecule, the nitrogen atom forms three sigma bonds with three hydrogen atoms and has one lone pair of electrons. The presence of the lone pair and the three sigma bonds leads to the formation of four equivalent hybrid orbitals, resulting in
hybridization.
20. The correct answer is a) Trigonal planar. The geometry of
is trigonal planar. In the
molecule, the boron atom forms three sigma bonds with three fluorine atoms and has no lone pairs of electrons. The three sigma bonds and the absence of lone pairs result in a trigonal planar geometry.
Clique para avaliar:
