Pergunta
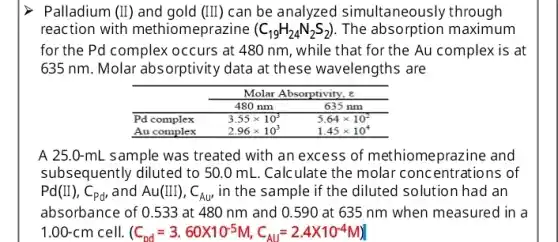
Palladium (II) and gold (III) can be analyzed simultaneously through reaction with methiomeprazine (mathrm(C)_(19) mathrm(H)_(24) mathrm(~N)_(2) mathrm(~S)_(2)) . The absorption maximum for the Pd complex occurs at 480 mathrm(~nm) , while that for the Au complex is at 635 mathrm(~nm) . Molar absorptivity data at these wavelengths are & multicolumn(2)(c)( Molar Absorptivity, boldsymbol(e) ) cline ( 2 - 3 ) & 480 mathrm(~nm) & 635 mathrm(~nm) Pd complex & 3.55 times 10^3 & 5.64 times 10^2 Au complex & 2.96 times 10^3 & 1.45 times 10^4 A 25.0-mL sample was treated with an excess of methiomeprazine and subsequently diluted to 50.0 mathrm(~mL) . Calculate the molar concentrations of mathrm(Pd)(mathrm(II)), mathrm(C)_(mathrm(Pd) mathrm(d)) and mathrm(Au)(mathrm(III)), mathrm(C)_(mathrm(Au)) , in the sample if the diluted solution had an absorbance of 0.533 at 480 mathrm(~nm) and 0.590 mathrm(at) 635 mathrm(~nm) when measured in a 1.00-mathrm(cm) cell. (mathrm(C)_(mathrm(Dd))=3.60 times 10^-5 mathrm(M), mathrm(C)_(mathrm(Au))=2.4 times 10^-4 mathrm(M))
Solução
 Verification of experts
Verification of experts4.0269 Voting
QuitériaVeterano · Tutor por 9 anos
Responder
### C_{\text{Pd}} = 3.00 \times 10^{-6} \, \text{M}, \, C_{\text{Au}} = 8.14 \times 10^{-5} \, \text{M}
Explicação
## Step1: Calculate the Molar Concentration of Pd Complex
### To find the molar concentration of the Palladium (Pd) complex, we use Beer-Lambert Law, which states that A = \varepsilon \cdot c \cdot l , where A is absorbance, \varepsilon is molar absorptivity, c is concentration, and l is the path length. Rearranging gives us c = \frac{A}{\varepsilon \cdot l} . For the Pd complex at 480 nm:
- Absorbance A = 0.533
- Molar absorptivity \varepsilon = 3.55 \times 10^5 \, \text{L mol}^{-1} \text{cm}^{-1}
- Path length l = 1.00 \, \text{cm}
Substituting these values into the equation:
c_{\text{Pd}} = \frac{0.533}{3.55 \times 10^5 \cdot 1.00}
## Step2: Calculate the Molar Concentration of Au Complex
### Similarly, for the Gold (Au) complex at 635 nm, we apply the same formula:
- Absorbance A = 0.590
- Molar absorptivity \varepsilon = 1.45 \times 10^4 \, \text{L mol}^{-1} \text{cm}^{-1}
Substituting these values into the equation:
c_{\text{Au}} = \frac{0.590}{1.45 \times 10^4 \cdot 1.00}
## Step3: Adjust for Dilution
### Since the sample was diluted from 25.0 mL to 50.0 mL, we need to account for this dilution factor of 2. Therefore, the final concentrations will be:
C_{\text{Pd}} = c_{\text{Pd}} \times 2
C_{\text{Au}} = c_{\text{Au}} \times 2
Now, let's calculate the values.
### Calculation for Pd:
c_{\text{Pd}} = \frac{0.533}{3.55 \times 10^5} \approx 1.50 \times 10^{-6} \, \text{M}
Thus,
C_{\text{Pd}} = 1.50 \times 10^{-6} \times 2 = 3.00 \times 10^{-6} \, \text{M}
### Calculation for Au:
c_{\text{Au}} = \frac{0.590}{1.45 \times 10^4} \approx 4.07 \times 10^{-5} \, \text{M}
Thus,
C_{\text{Au}} = 4.07 \times 10^{-5} \times 2 = 8.14 \times 10^{-5} \, \text{M}
### To find the molar concentration of the Palladium (Pd) complex, we use Beer-Lambert Law, which states that A = \varepsilon \cdot c \cdot l , where A is absorbance, \varepsilon is molar absorptivity, c is concentration, and l is the path length. Rearranging gives us c = \frac{A}{\varepsilon \cdot l} . For the Pd complex at 480 nm:
- Absorbance A = 0.533
- Molar absorptivity \varepsilon = 3.55 \times 10^5 \, \text{L mol}^{-1} \text{cm}^{-1}
- Path length l = 1.00 \, \text{cm}
Substituting these values into the equation:
c_{\text{Pd}} = \frac{0.533}{3.55 \times 10^5 \cdot 1.00}
## Step2: Calculate the Molar Concentration of Au Complex
### Similarly, for the Gold (Au) complex at 635 nm, we apply the same formula:
- Absorbance A = 0.590
- Molar absorptivity \varepsilon = 1.45 \times 10^4 \, \text{L mol}^{-1} \text{cm}^{-1}
Substituting these values into the equation:
c_{\text{Au}} = \frac{0.590}{1.45 \times 10^4 \cdot 1.00}
## Step3: Adjust for Dilution
### Since the sample was diluted from 25.0 mL to 50.0 mL, we need to account for this dilution factor of 2. Therefore, the final concentrations will be:
C_{\text{Pd}} = c_{\text{Pd}} \times 2
C_{\text{Au}} = c_{\text{Au}} \times 2
Now, let's calculate the values.
### Calculation for Pd:
c_{\text{Pd}} = \frac{0.533}{3.55 \times 10^5} \approx 1.50 \times 10^{-6} \, \text{M}
Thus,
C_{\text{Pd}} = 1.50 \times 10^{-6} \times 2 = 3.00 \times 10^{-6} \, \text{M}
### Calculation for Au:
c_{\text{Au}} = \frac{0.590}{1.45 \times 10^4} \approx 4.07 \times 10^{-5} \, \text{M}
Thus,
C_{\text{Au}} = 4.07 \times 10^{-5} \times 2 = 8.14 \times 10^{-5} \, \text{M}
Clique para avaliar:
