Pergunta
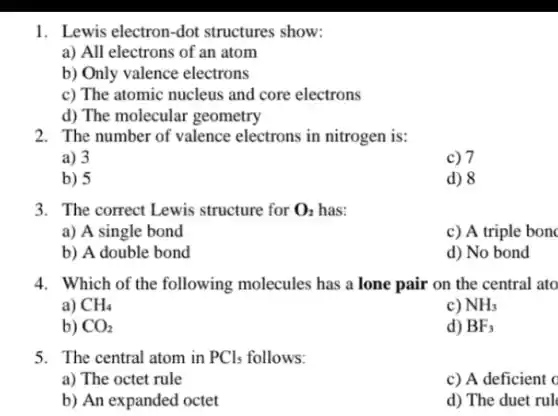
1. Lewis electron-dot structures show: a) All electrons of an atom b) Only valence electrons c) The atomic nucleus and core electrons d) The molecular geometry 2. The number of valence electrons in nitrogen is: a) 3 c) 7 b) 5 d) 8 3. The correct Lewis structure for O_(2) has: a) A single bond c) A triple bon b) A double bond d) No bond 4. Which of the following molecules has a lone pair on the central ato a) CH_(4) c) NH_(3) b) CO_(2) d) BF_(3) 5. The central atom in PCl_(5) follows: a) The octet rule c) A deficient c b) An expanded octet d) The duet rul
Solução
 Verification of experts
Verification of experts4.4203 Voting
CamilaElite · Tutor por 8 anos
Responder
1. b) Only valence electrons
2. b) 5
3. b) A double bond
4. c) NH_{3}
5. b) An expanded octet
2. b) 5
3. b) A double bond
4. c) NH_{3}
5. b) An expanded octet
Clique para avaliar:
